The First Fully Automatic Production Line In China
Sterile Production Line for Dissolving Microneedle Formulations
Youwe boasts a sprawling GMP-certified plant, spanning over 3,000 square meters, as well as cutting-edge research and development facilities, quality control laboratories, and a sterile production line dedicated to dissolving microneedle formulations. This expansion marks a significant stride toward broader commercial applications within the medical sector. Our current research endeavors encompass a spectrum of traditional Chinese medicine extracts and large molecular drugs, including peptides, proteins, recombinant protein vaccines, and more. These groundbreaking formulations hold promise in diverse domains such as central nervous system disease treatment, pain management, hormone metabolism disease treatment, and vaccine administration. At Youwe, we are committed to delivering comprehensive, professional, and rigorously quality-assured dissolving microneedle drug products, complemented by top-tier medical device CRO+CDMO services.
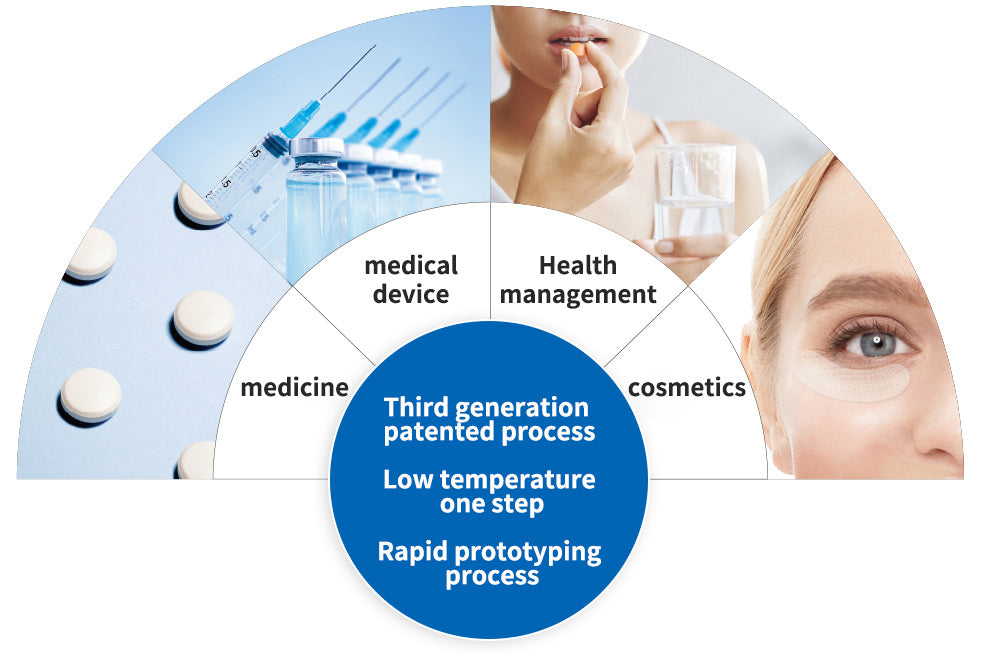
Third generation soluble microneedle technology manufacturing process
Can be widely used in the fields of medicine, medical equipment, general health and medical beauty
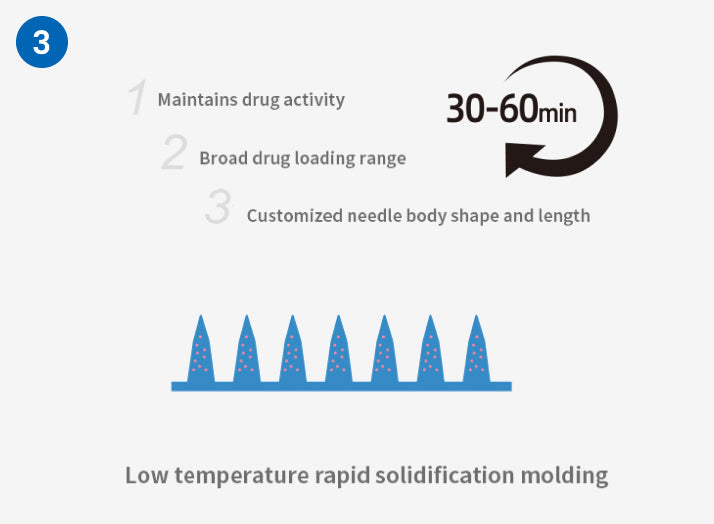
One Step LT-MN Technology® Low Temperature One Step Rapid Prototyping Process
Independent innovation and research and development technology
Compared with the traditional mold method and stretching method preparation process, which have disadvantages such as difficulty in maintaining drug activity, limited crystal morphology, and poor stability between batches, Excellent Microorganisms independently developed the third generation soluble microneedle process - One Step LT -MN Technology ® process can maintain the activity of ingredients in normal or low-temperature environments while simultaneously improving the industrial technology capabilities of soluble microneedles in multiple dimensions, and improving flexible customization and industrialization capabilities. In addition, it can also achieve precise drug loading in segmented microneedles. This breakthrough development can meet the requirements for precise and quantitative transdermal delivery of drugs and broaden the application scope of microneedle transdermal drug delivery forms.
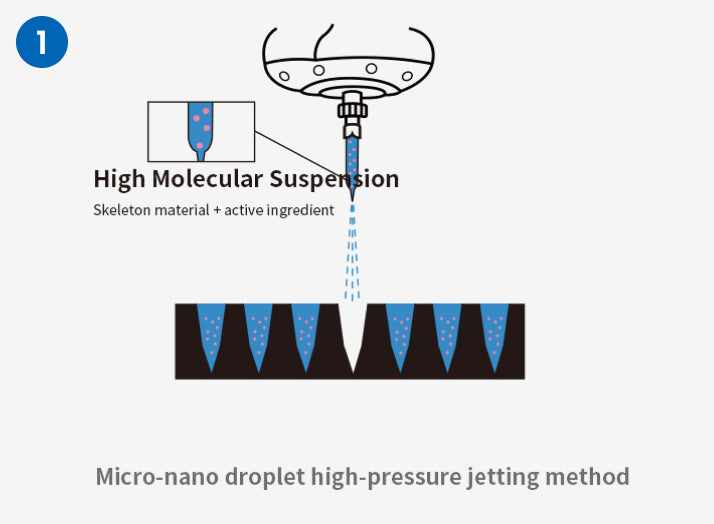
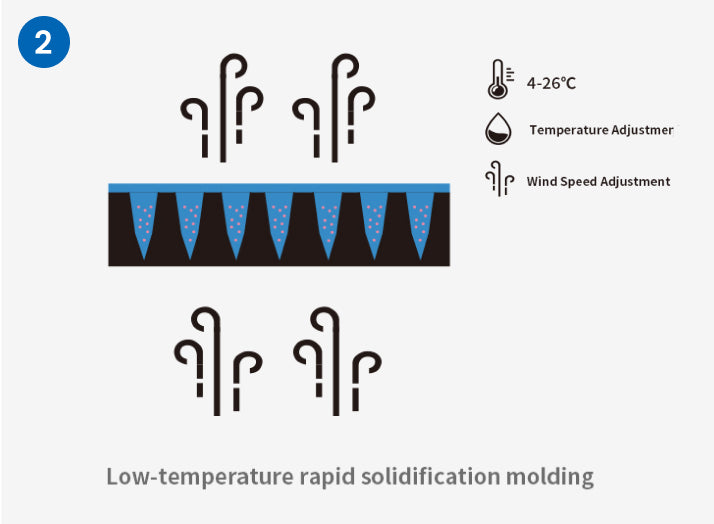
Prominent Advantages of Independent Research and Development Process
Comprehensive quality control system compliant with cosmetics GMP and ISO 22716 standards
The most cutting-edge production system in China, a fully automated intelligent production system, and flexible production. The highest industry standard, 10,000-level GMP production factory, passed ISO22716:2007/GMPC certification.



Used in cosmetics field
Solid Essence Micro Dot Matrix Patch Fully Automatic Production Line
Based on its independent research and development process, Umimicrobiology has built China's first fully automated production line in the cosmetics field to achieve mass production of microlattice patches. Adopt China's most cutting-edge production system, fully automated intelligent production system, and flexible production. Reaching the highest standards in the cosmetics industry, the 10,000-level GMP production factory has passed ISO22716:2007/GMPC certification and has a 2D/3D visual inspection system to ensure a yield rate of up to 98%.